Abstract
Introduction:
Many lymphoma patients receive high doses of glucocorticoids as part of standard therapy, and several recent observational studies have highlighted a possible risk of glucocorticoid-induced osteoporosis (GIO) and excess bone fracture risk. As a substantial fraction of lymphoma patients become long-term survivors, studies that focus on mitigating the negative effects of treatment toxicities on survivorship are important. The objective of the SIESTA trial was to determine if primary prophylaxis with oral alendronate (ALN) is safe and effective against (GIO) in lymphoma patients.
Methods:
SIESTA was a single-center randomized and double-blinded phase 2 study performed at the Department of Hematology, Aalborg University Hospital, Denmark, enrolling lymphoma patients that were planned for glucocorticoid-containing chemotherapy regimens such as (R-)CVP and all variants of (R)-CHOP. Patients were randomized to weekly oral ALN 70mg or placebo with a treatment duration of 52 weeks. Study assessments included bone mineral density (BMD) measurements at baseline, after completion of chemotherapy at 4-6 months (EOT), and after 12 months, as well as vertebral fracture assessment (VFA) at baseline and at 12 months. The primary study endpoint was change in lumbar spine T-score from baseline to 12 months. Key secondary endpoints were change in T-score from baseline to 12 months at total hip and femoral neck levels, vertebral compression fractures and early T-score changes. Biomarker analyses were exploratory. The target recruitment was 60 patients in a three-year period.
Results:
A total of 59 patients (36 Diffuse large B-cell lymphoma, 15 follicular lymphoma, 8 other) were enrolled with 22 of 30 patients in the ALN arm and 23 of 29 patients in the placebo arm completing the study (efficacy group). Patient characteristics in the two arms were balanced with exception of more advanced stage diseases (Ann Arbor stage III-IV) in the ALN arm (70·0% vs 41·4%), and a lower median baseline T-score at the lumbar spine in the ALN arm (median T-score -0·6 vs 1·0).
Patients in the ALN group received a median of 3,291 mg prednisone versus 3,398 mg for the placebo group.
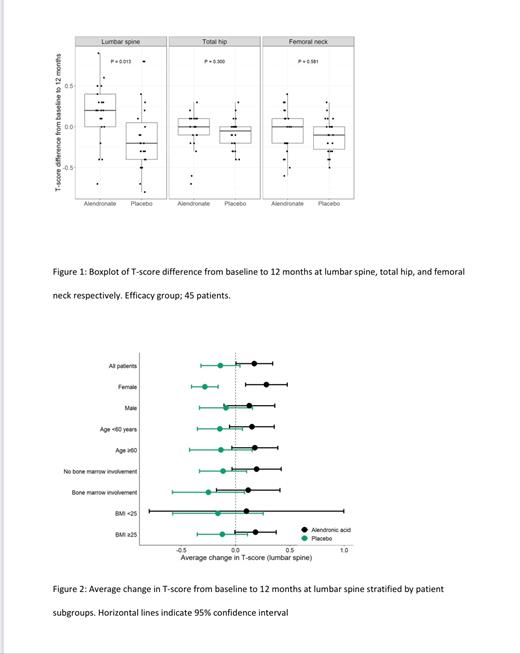
Median change in T-score from baseline to 12 months at the lumbar spine level (primary endpoint) was +0·2 for the ALN arm and -0·2 for the placebo arm (P=0·013) (figure 1), with stronger effect for female patients (median change; ALN +0·25; placebo -0·25) (figure 2). ALN had no effect on BMD for total hip (P=0·30) and femoral neck (P=0·58) at 12 months (figure 1). ALN had no significant early effects on BMD for any measured sites (4-6 months). No new fractures were observed.
Nine patients experienced AEs related to the upper gastro-intestinal (GI) system (7 grade 1-2, 2 grade 3-4) with 5 AEs being assessed as related to the study treatment (3 in the ALN group and 2 in the placebo group). One patient (placebo group) discontinued study treatment due to upper GI AE (bleeding ulcer).
Biomarker analyses (C-terminal telopeptide cross links (CTX) as marker of bone resorption and N-terminal propeptides of collagen type 1 (P1NP) as marker for bone formation) showed reduced bone resorption for ALN treated patients opposed to the placebo treated patients. From baseline to EOT the mean change in CTX was -0.17 in the ALN group and 0.10 in the placebo group respectively (P<0.001). From baseline to 12 months the mean change in CTX was -0.19 in the ALN group and 0.00 in the placebo group respectively (P=0.002).
Interpretation:
ALN was a safe and effective primary prophylaxis against GIO in lymphoma patients planned for glucocorticoid-containing chemotherapy regimens. The treatment effects were clinically meaningful across all patient subgroups, but the largest effect size was observed in females. Biomarker analyses supported reduced bone resorption for ALN treated patients.
Vestergaard: Novo Nordisk Foundation: Other: Head of Research at Steno Diabetes Center North Jutland funded by the Novo Nordisk Foundation, Research Funding. El-Galaly: Abbvie: Other: Speakers fee; ROCHE Ltd: Ended employment in the past 24 months.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal